Cowingene Respiratory Syncytial Virus Detection Kit (Liquid)-HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|PCR-based assay,High sensitivity
The Cowingene Respiratory Syncytial Virus (RSV) Detection Kit (Liquid) represents a significant advancement in molecular diagnostics, offering rapid, accurate, and reliable detection of RSV, a major cause of respiratory infections in infants, young children, and immunocompromised individuals. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., this kit is designed to meet the stringent demands of clinical laboratories and healthcare providers worldwide. Below, we explore its core features, technical specifications, applications, and the company's commitment to innovation in diagnostic technologies.
Product Overview
The Cowingene RSV Detection Kit (Liquid) is a polymerase chain reaction (PCR)-based assay specifically engineered to detect the presence of Respiratory Syncytial Virus (RSV) in clinical specimens. RSV is a leading cause of bronchiolitis and pneumonia in young children, with global annual infections estimated at 64 million cases, resulting in approximately 130,000 deaths in children under five years old (WHO, 2023). The kit's ability to identify RSV in a timely manner is critical for early intervention and effective treatment strategies.
The product is validated for use with multiple specimen types, including nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, ensuring broad applicability across diverse clinical scenarios. Its liquid format simplifies handling and reduces the risk of contamination, making it a preferred choice for high-throughput laboratories.
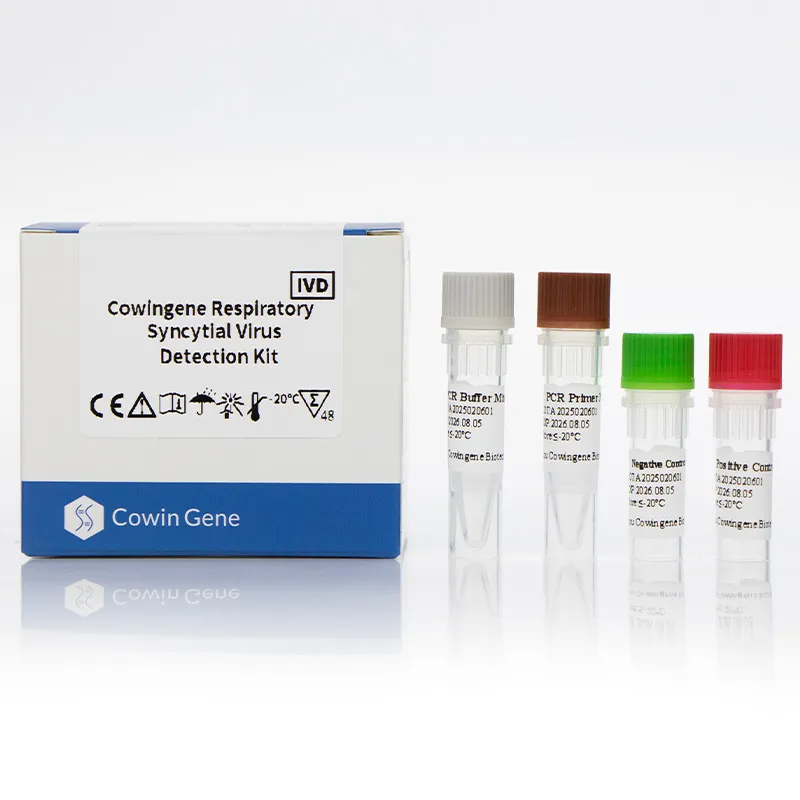
Read More About respiratory pathogen panel pcr
Key Features and Advantages
The Cowingene RSV Detection Kit combines cutting-edge technology with user-friendly design to deliver exceptional performance. Below are its standout features:
- High Sensitivity and Specificity: The kit utilizes advanced PCR techniques to detect even low viral loads, minimizing false negatives and ensuring accurate diagnosis.
- Fast Turnaround Time: Results are typically available within 2–3 hours, enabling timely clinical decision-making.
- Comprehensive Validation: The product is validated for multiple specimen types, ensuring consistency and reliability across different sample matrices.
- Minimal Hands-On Time: The liquid format reduces the need for complex preparation steps, streamlining workflows in busy laboratories.
- Compliance with Industry Standards: The kit adheres to rigorous quality control protocols, aligning with global standards for diagnostic testing.
For healthcare professionals, these advantages translate into improved patient outcomes, reduced hospitalization rates, and more efficient resource allocation. The kit's reliability is further reinforced by its alignment with the National Institute of Standards and Technology (NIST) guidelines, which emphasize precision and traceability in diagnostic measurements.
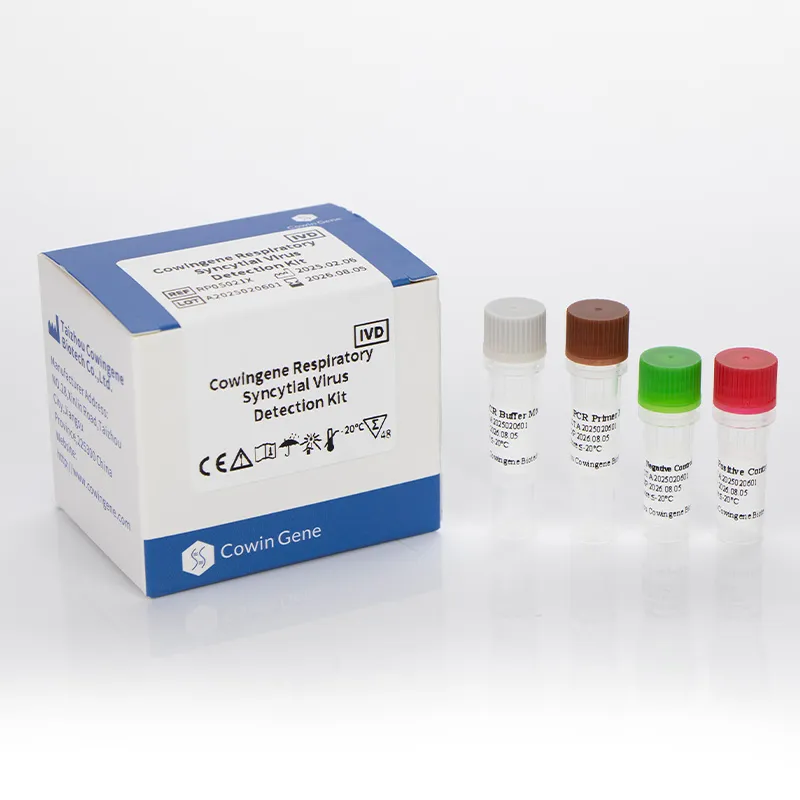
Read More About respiratory panel test for
Technical Specifications
The Cowingene RSV Detection Kit is designed to meet the highest standards of performance and usability. Below is a detailed specification table:
| Parameter | Details |
|---|---|
| Test Type | PCR-based detection of RSV |
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Specimen Types | Nasopharyngeal swab, nasopharyngeal aspirate, bronchoalveolar lavage, throat swab |
| Assay Format | Liquid reagent for ease of use |
| Sensitivity | ≥ 100 copies/mL (validated by independent testing) |
| Specificity | ≥ 99% (no cross-reactivity with common respiratory pathogens) |
| Turnaround Time | 2–3 hours (depending on laboratory equipment) |
| Storage Conditions | -20°C; stable for 12 months from the date of manufacture |
These specifications highlight the kit's ability to deliver consistent, high-quality results under varying laboratory conditions. The product page provides further details on validation studies and performance metrics.
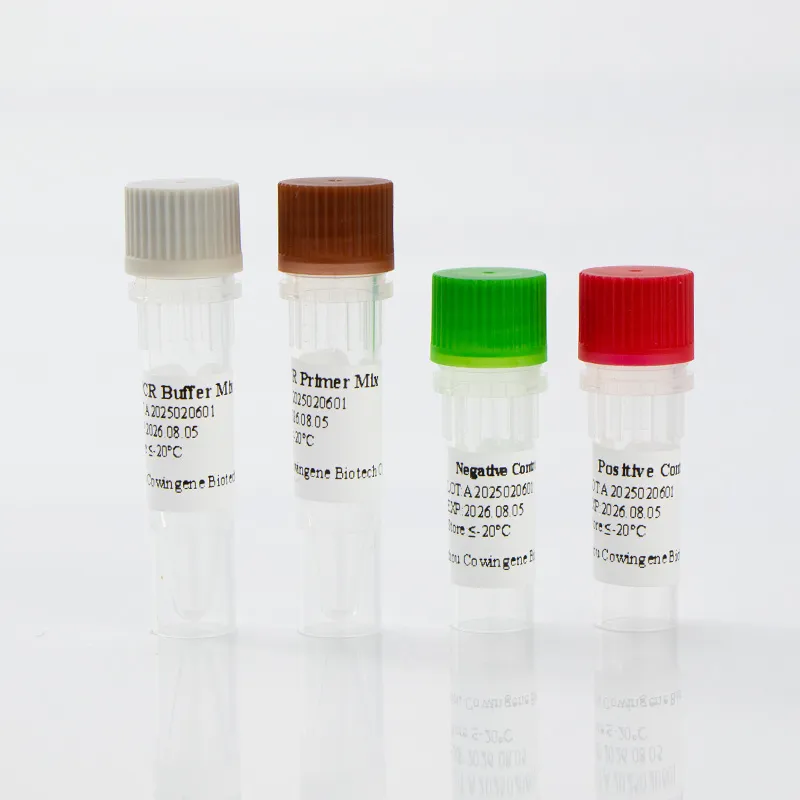
Read More About respiratory panel test for
Applications in Clinical and Research Settings
The Cowingene RSV Detection Kit is widely applicable in both clinical and research environments. In clinical settings, it is used to:
- Diagnose RSV Infections: Rapid identification of RSV in patients presenting with respiratory symptoms, such as coughing, wheezing, and fever.
- Monitor Outbreaks: Assist public health agencies in tracking RSV transmission patterns and implementing targeted interventions.
- Support Treatment Decisions: Guide clinicians in prescribing antiviral therapies and managing complications like pneumonia or bronchiolitis.
In research settings, the kit is employed to:
- Investigate Viral Pathogenesis: Study the mechanisms of RSV replication and its interaction with host immune systems.
- Evaluate Vaccine Efficacy: Assess the performance of experimental vaccines in preventing RSV infections.
- Develop Diagnostic Tools: Serve as a benchmark for developing new assays and improving existing diagnostic technologies.
The versatility of the kit makes it an indispensable tool for laboratories focused on infectious disease research and public health surveillance.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
Founded in 2010, HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic reagents and molecular testing solutions. Based in Hebei Province, China, the company has established itself as a trusted partner for healthcare providers, research institutions, and diagnostic laboratories worldwide. With a focus on innovation, quality, and customer satisfaction, HEBEI YOWIN has consistently delivered products that meet the evolving needs of the global market.
The company's commitment to excellence is reflected in its adherence to international standards, including ISO 13485 for medical device quality management systems and CE marking for compliance with European Union regulations. HEBEI YOWIN's state-of-the-art facilities and skilled team of scientists and engineers ensure that its products are developed with the highest level of precision and reliability.
As part of its ongoing efforts to advance diagnostic technologies, HEBEI YOWIN collaborates with academic institutions and industry partners to explore new applications for molecular testing. The Cowingene RSV Detection Kit is a testament to the company's dedication to addressing critical public health challenges through innovative solutions.
For more information about HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., visit their company page.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) exemplifies the intersection of cutting-edge technology and clinical necessity. Its high sensitivity, rapid results, and broad applicability make it an essential tool for diagnosing RSV infections and improving patient outcomes. By aligning with the standards set by organizations like the National Institute of Standards and Technology (NIST), HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. ensures that its products meet the rigorous demands of modern healthcare.
As the global healthcare landscape continues to evolve, the importance of reliable diagnostic tools like the Cowingene RSV Detection Kit cannot be overstated. With its commitment to innovation and quality, HEBEI YOWIN is well-positioned to lead the way in advancing molecular diagnostics for respiratory pathogens.
References
1. National Institute of Standards and Technology (NIST). (n.d.). Standards and Measurements. Retrieved from https://www.nist.gov/
2. World Health Organization (WHO). (2023). Respiratory Syncytial Virus (RSV) Fact Sheet. Retrieved from https://www.who.int/
3. HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. (n.d.). Cowingene Respiratory Syncytial Virus Detection Kit (Liquid). Retrieved from https://www.cowingene.com/cowingene-respiratory-syncytial-v-nl.html
