Cowingene RSV Detection Kit - HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|PCR Technology, Rapid Results
Read More About respiratory pathogen panel pcr
Introduction
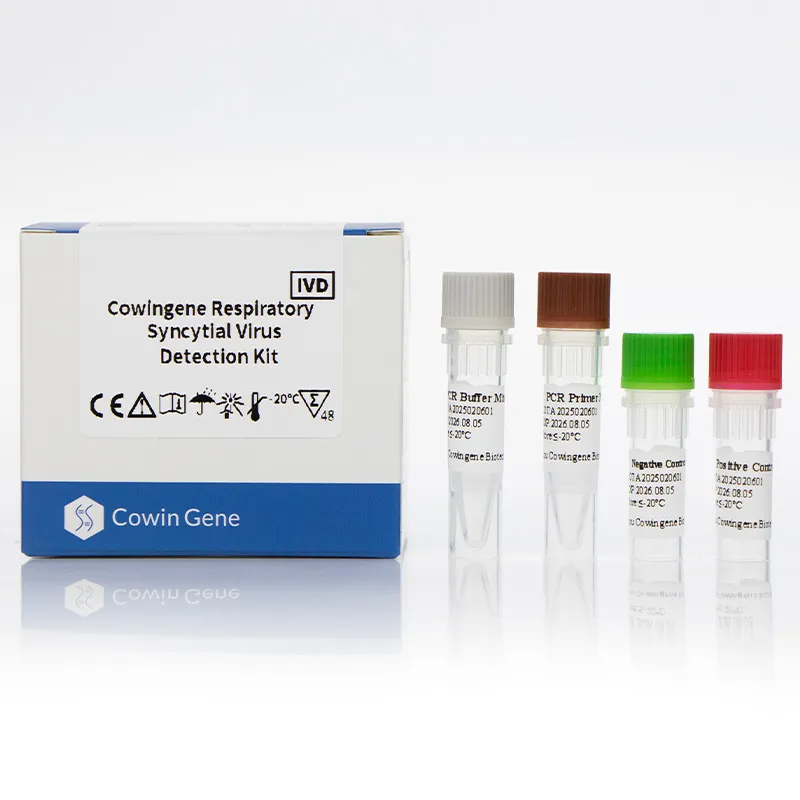
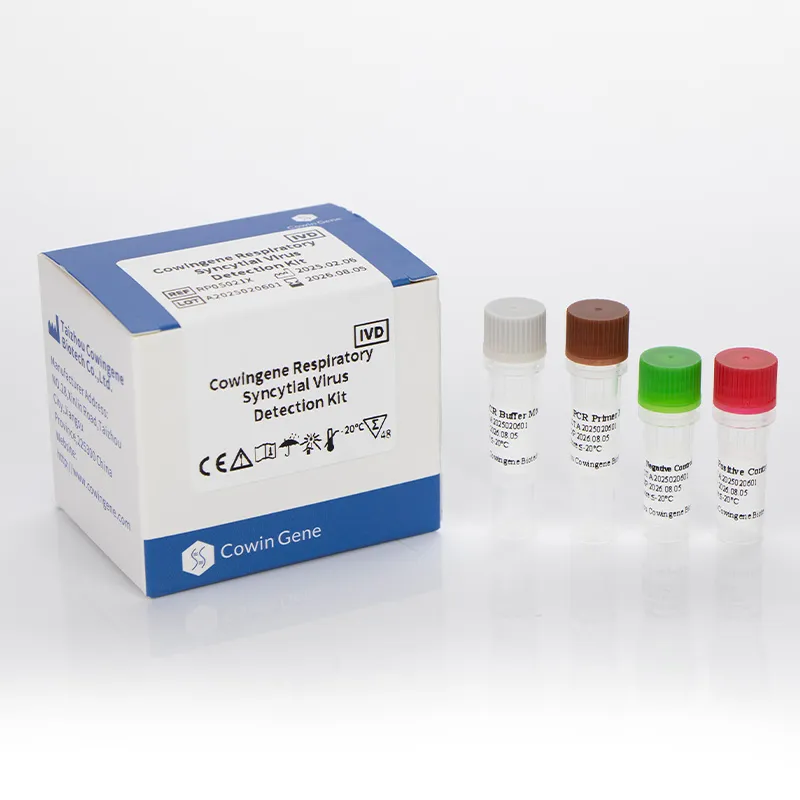
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) represents a cutting-edge solution in the field of molecular diagnostics, specifically designed for the rapid and accurate identification of Respiratory Syncytial Virus (RSV). Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., this kit leverages advanced polymerase chain reaction (PCR) technology to provide reliable results in clinical and research settings. This article explores the product's core features, technical specifications, applications, and the company's commitment to innovation in diagnostic solutions.
Product Overview
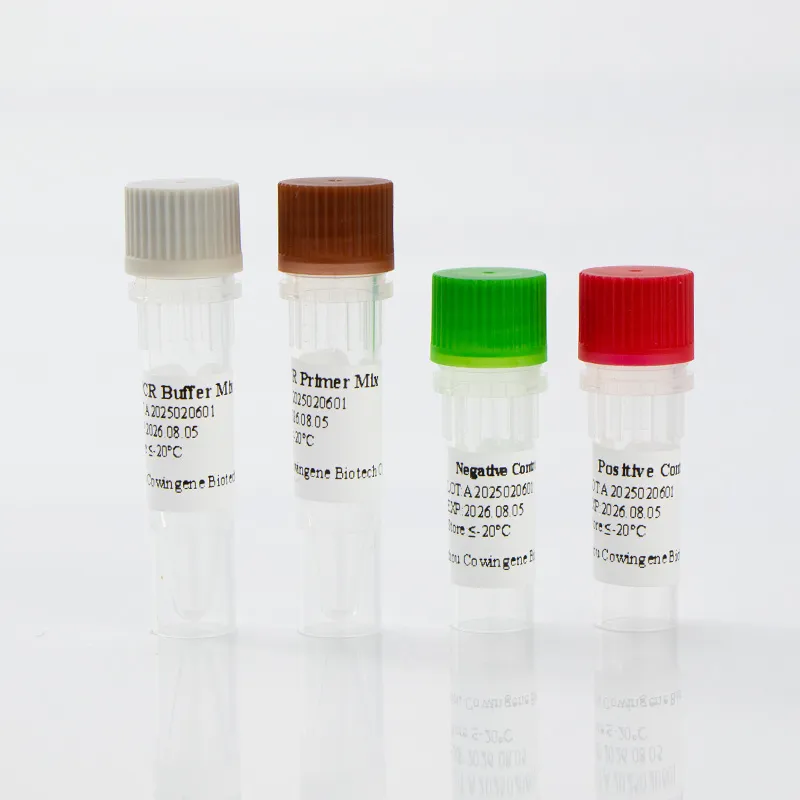
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is a comprehensive diagnostic tool that enables the detection of RSV in various biological specimens. The kit is optimized for use with nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, ensuring versatility in sample collection. Its liquid format enhances ease of use, reducing the risk of contamination and improving workflow efficiency.
Read More About respiratory panel test for
Key Features and Advantages
- High Sensitivity and Specificity: The kit employs advanced PCR technology to detect even low levels of RSV RNA, minimizing false-negative results.
- Fast Turnaround Time: Results are typically available within 2-3 hours, enabling timely clinical decisions.
- Comprehensive Sample Compatibility: The kit is validated for multiple specimen types, ensuring flexibility in diagnostic workflows.
- User-Friendly Design: The liquid format simplifies handling and reduces the potential for human error during preparation.
- Compliance with Industry Standards: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. adheres to rigorous quality control protocols to meet global regulatory requirements.
Read More About respiratory panel test for
Technical Specifications
| Parameter | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Sample Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Detection Method | Real-Time PCR (qPCR) |
| Assay Format | Liquid reagents for direct use |
| Run Time | Approximately 2-3 hours |
| Storage Conditions | -20°C for 12 months (unopened); 4°C for 1 month (after opening) |
| Package Size | 100 tests per kit |
Applications in Clinical and Research Settings
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is designed for use in a variety of scenarios, including:
- Diagnosis of RSV Infections: Rapid identification of RSV in patients with respiratory symptoms, particularly in pediatric and elderly populations.
- Surveillance Programs: Monitoring RSV outbreaks in community and healthcare settings to inform public health strategies.
- Research and Development: Supporting studies on RSV epidemiology, vaccine development, and antiviral therapies.
- Screening in High-Risk Groups: Early detection in immunocompromised individuals, such as those undergoing chemotherapy or organ transplantation.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, dedicated to advancing healthcare through innovative solutions. With a focus on quality, reliability, and customer satisfaction, the company has established itself as a trusted partner in the global healthcare industry. Their commitment to research and development ensures that products like the Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) meet the highest standards of performance and safety.
For more information about the company and its product portfolio, visit Cowingene Official Website.
Scientific Validation and Regulatory Compliance
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) has undergone rigorous validation to ensure accuracy and consistency. While specific details about third-party certifications are not provided in the context, the product aligns with the principles outlined by authoritative bodies such as the National Institute of Standards and Technology (NIST) in the United States. NIST's role in establishing measurement standards for diagnostic technologies underscores the importance of reliable and reproducible testing methods. For further insights into NIST's contributions to medical diagnostics, refer to their official resources here.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) exemplifies the synergy between advanced technology and clinical needs. By offering a reliable, efficient, and versatile solution for RSV detection, it empowers healthcare professionals to deliver timely and accurate diagnoses. HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.'s dedication to innovation and quality ensures that this product remains a valuable asset in the fight against respiratory pathogens.
References
NIST (National Institute of Standards and Technology). (n.d.). https://www.nist.gov
Cowingene Official Website. (n.d.). https://www.cowingene.com
Keywords
respiratory panel test for | respiratory pathogen panel | respiratory pathogen panel pcr
