Cowingene RSV Detection Kit - HEBEI YOWIN | PCR, Rapid Testing
Respiratory Syncytial Virus (RSV) is a leading cause of lower respiratory tract infections in infants and young children, with significant global health implications. The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) offers a cutting-edge solution for rapid, accurate, and reliable RSV detection. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., this diagnostic tool is designed to meet the demands of modern clinical laboratories and healthcare providers. This article explores the product's features, technical specifications, applications, and the company's commitment to innovation.
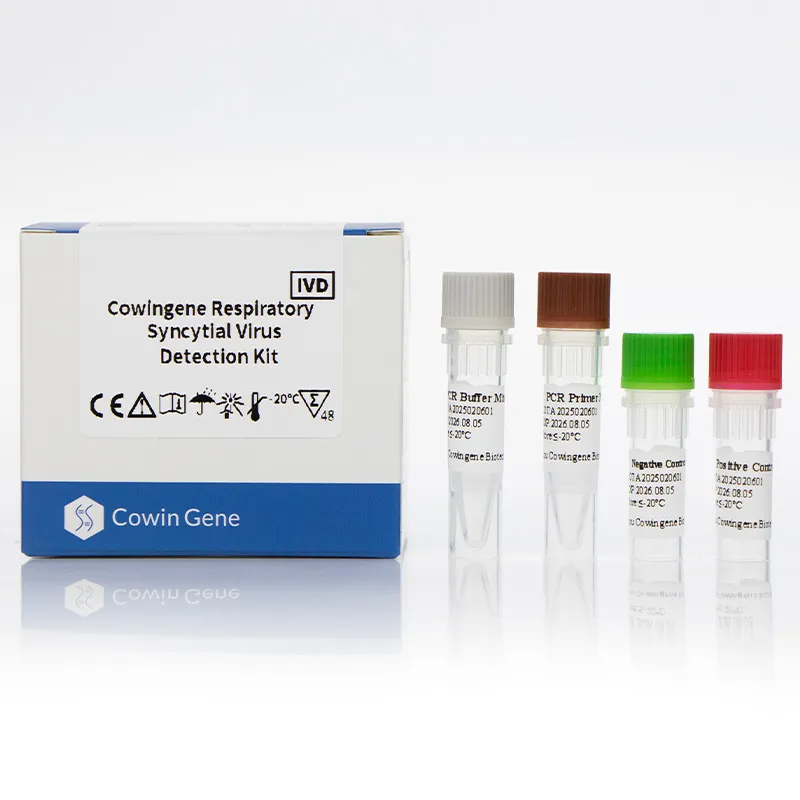
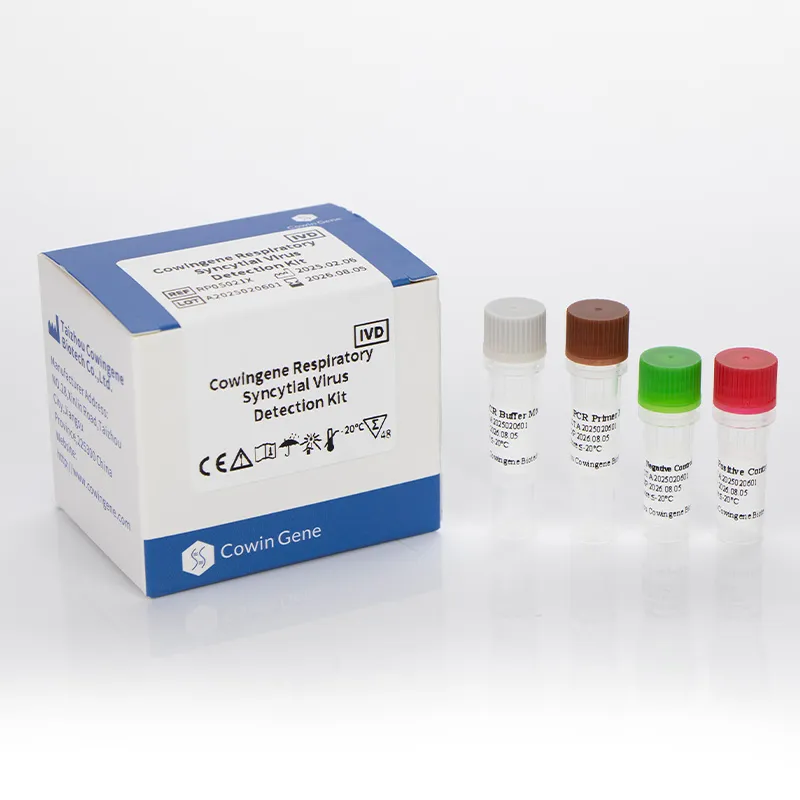
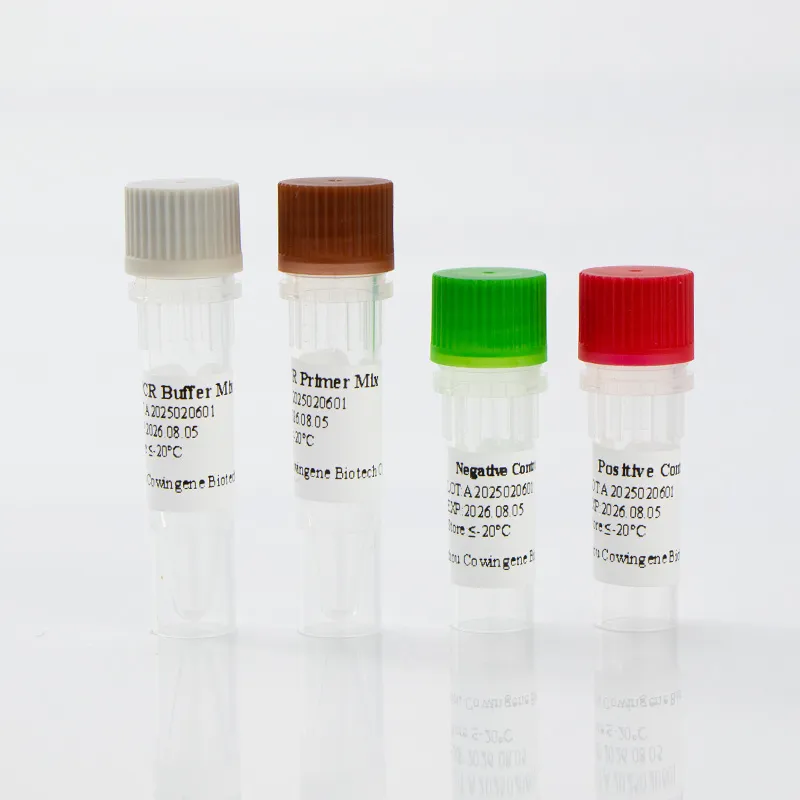
Product Overview
The Cowingene RSV Detection Kit (Liquid) is a molecular diagnostic test designed for the qualitative detection of Respiratory Syncytial Virus (RSV) in clinical specimens. This kit utilizes advanced PCR (Polymerase Chain Reaction) technology to identify RSV genetic material, ensuring high sensitivity and specificity. The product is validated for use with a range of specimen types, including nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, making it versatile for various clinical settings.
Key Features and Advantages
1. High Sensitivity and Specificity
The kit employs a respiratory pathogen panel PCR approach, which targets specific genetic sequences of RSV. This method minimizes cross-reactivity with other respiratory pathogens, ensuring accurate identification of RSV. The kit's design allows for the detection of even low viral loads, making it ideal for early diagnosis and intervention.
2. Rapid and Efficient Workflow
With a streamlined protocol, the Cowingene RSV Detection Kit reduces the time required for sample processing and analysis. The liquid reagent format simplifies handling, reducing the risk of contamination and ensuring consistency across tests. This efficiency is critical in high-throughput clinical laboratories where timely results are essential.
3. Comprehensive Validation
The kit has been validated for use with multiple specimen types, including respiratory panel test for nasopharyngeal swabs and bronchoalveolar lavage. This broad validation ensures that healthcare providers can use the kit across diverse patient populations and clinical scenarios, enhancing its utility in both hospital and outpatient settings.
4. User-Friendly Design
Engineered for ease of use, the kit includes all necessary reagents and components in a single, ready-to-use format. This design reduces the need for additional equipment and minimizes the potential for human error. The clear, step-by-step instructions further simplify the testing process, making it accessible to laboratory personnel with varying levels of expertise.
Technical Specifications
| Parameter | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Sample Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Test Format | Qualitative Detection |
| Technology | PCR (Polymerase Chain Reaction) |
| Kit Components | Lyophilized reagents, liquid master mix, positive/negative controls |
| Storage Conditions | -20°C for long-term storage; 4°C for short-term use |
| Shelf Life | 12 months from the date of manufacture |
Applications and Use Cases
The Cowingene RSV Detection Kit is designed for use in a variety of clinical and research settings, including:
- Diagnostic Laboratories: Rapid identification of RSV in patients presenting with respiratory symptoms, enabling timely treatment and isolation protocols.
- Public Health Surveillance: Monitoring RSV outbreaks and tracking viral prevalence to inform public health strategies.
- Research Institutions: Studying RSV epidemiology, viral evolution, and the effectiveness of antiviral therapies.
- Outpatient Clinics: Providing point-of-care testing for patients with suspected RSV infections, reducing the need for hospitalization.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, dedicated to advancing healthcare through innovative solutions. With a focus on quality, reliability, and customer satisfaction, the company has established itself as a trusted partner for laboratories and healthcare providers worldwide. HEBEI YOWIN's commitment to research and development ensures that its products meet the highest standards of performance and safety.
As a company that prioritizes respiratory panel test for accuracy and efficiency, HEBEI YOWIN has consistently delivered products that address the evolving needs of the healthcare industry. The Cowingene RSV Detection Kit exemplifies this dedication, offering a reliable tool for RSV diagnosis in both clinical and research environments.
Industry Standards and Compliance
The Cowingene RSV Detection Kit adheres to international standards for diagnostic testing, ensuring compliance with regulatory requirements. While specific certifications (e.g., CE, FDA) are not mentioned in the context, the product's design and validation align with the principles outlined by organizations such as the National Institute of Standards and Technology (NIST). NIST plays a critical role in developing measurement standards that underpin the accuracy and reliability of diagnostic technologies, as highlighted in its mission to "advance innovation and industrial competitiveness" through measurement science (NIST, 2025).
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) represents a significant advancement in the field of respiratory pathogen detection. With its high sensitivity, rapid workflow, and comprehensive validation, this kit provides healthcare professionals with a reliable tool for diagnosing RSV infections. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., a company committed to innovation and quality, the product underscores the importance of precision in modern diagnostics. As the demand for efficient and accurate diagnostic solutions continues to grow, the Cowingene RSV Detection Kit stands out as a valuable asset for laboratories and healthcare providers worldwide.
References
National Institute of Standards and Technology (NIST). (2025). Advancing Innovation and Industrial Competitiveness. Retrieved from https://www.nist.gov/
