Respiratory Panel Test for RSV - HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|PCR Detection, Rapid Results
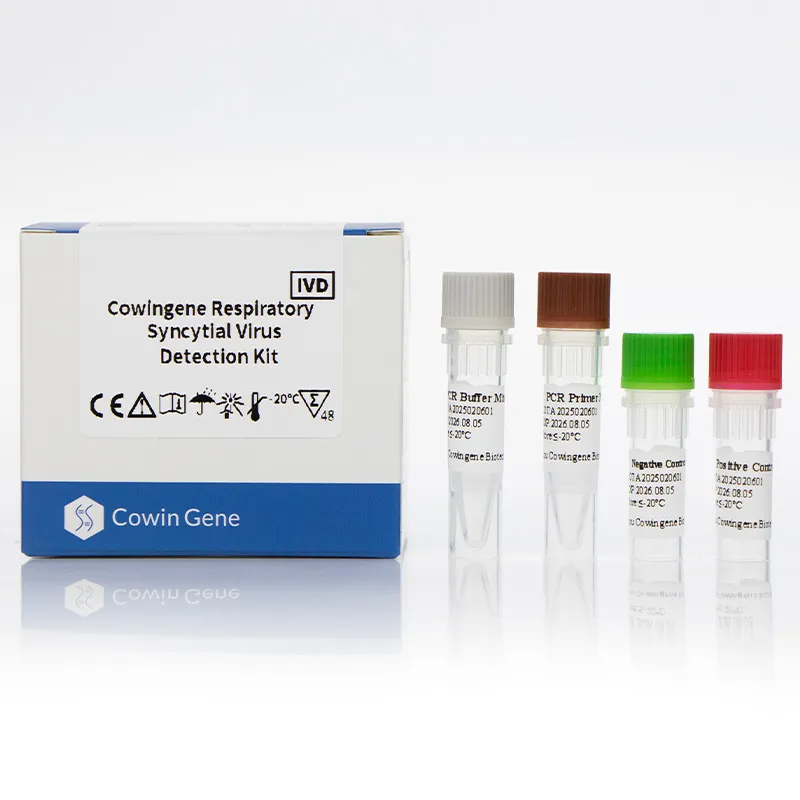
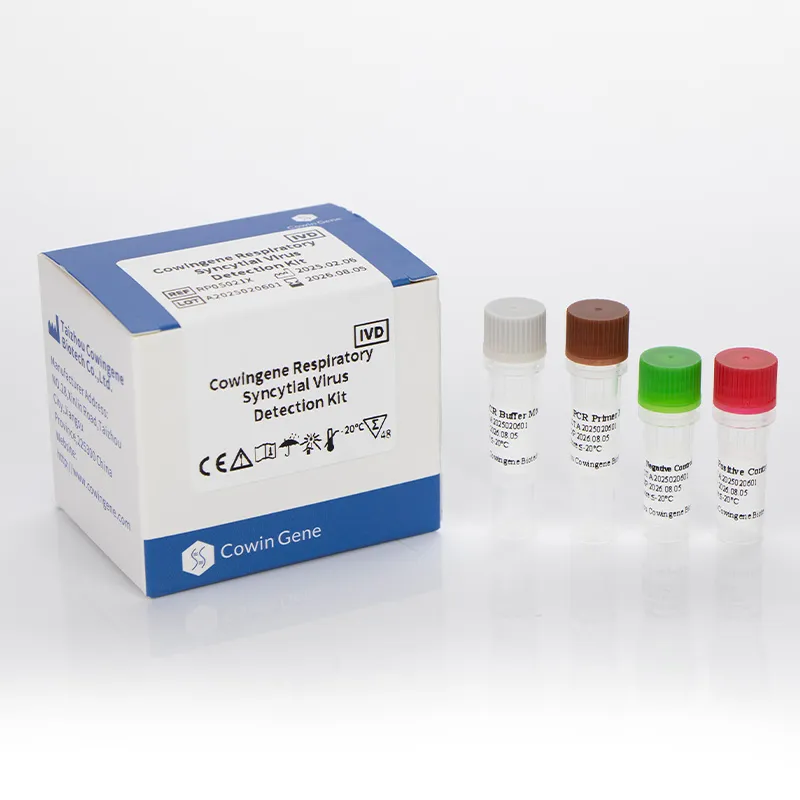
Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is a cutting-edge diagnostic tool designed to detect Respiratory Syncytial Virus (RSV) in clinical samples. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., this kit combines advanced molecular biology techniques with user-friendly design to deliver rapid, accurate, and reliable results. This article provides an in-depth analysis of the product's features, technical specifications, applications, and the company's background.
Product Functionality and Key Features
The Respiratory Syncytial Virus Detection Kit is engineered to identify the presence of RSV in various biological specimens, including nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs. This versatility ensures its applicability across diverse clinical settings. The kit employs polymerase chain reaction (PCR) technology, a gold standard in molecular diagnostics, to amplify and detect RSV-specific genetic material with high sensitivity and specificity.
One of the standout features of this kit is its rapid turnaround time. Unlike traditional culture methods that may take days to yield results, the RSV detection kit provides actionable data within hours, enabling timely clinical decision-making. This is particularly critical in managing outbreaks and preventing the spread of RSV, which is a leading cause of respiratory infections in infants and young children.
The product also emphasizes user convenience. Its streamlined workflow reduces the need for complex laboratory equipment, making it suitable for point-of-care testing in resource-limited environments. Additionally, the kit includes all necessary reagents and controls, minimizing the risk of human error during the testing process.
Technical Specifications and Performance
The technical specifications of the RSV detection kit are designed to meet the rigorous demands of modern diagnostics. Below is a detailed overview of its key parameters:
| Parameter | Details |
|---|---|
| Sample Type | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Methodology | Real-time PCR |
| Detection Limit | 100 copies/mL (validated) |
| Turnaround Time | Less than 2 hours |
| Storage Conditions | -20°C for 12 months; 4°C for 6 months |
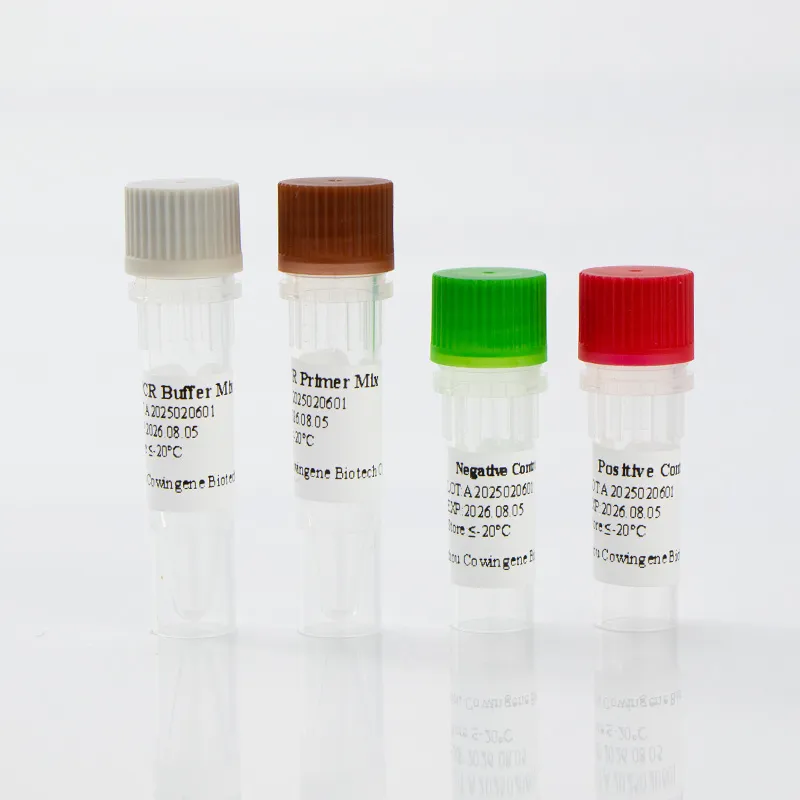
| Kit Components | PCR master mix, primers/probes, positive control, internal control |
These specifications highlight the kit's ability to deliver high sensitivity and specificity, which are crucial for accurate diagnosis. The inclusion of internal controls ensures the reliability of test results, while the validated detection limit guarantees consistent performance across different sample types.
Applications and Use Cases
The Respiratory Syncytial Virus Detection Kit is widely applicable in various healthcare settings. Its primary use is in clinical laboratories for the rapid identification of RSV in patients presenting with symptoms of respiratory tract infections. However, its utility extends beyond this scope:
- Outbreak Surveillance: The kit enables public health officials to monitor RSV activity and implement targeted interventions during seasonal outbreaks.
- Research and Development: Researchers can utilize the kit to study the epidemiology of RSV and evaluate the efficacy of new antiviral therapies.
- Point-of-Care Testing: The kit's portability and ease of use make it ideal for use in clinics, emergency departments, and mobile health units.
According to the National Institute of Standards and Technology (NIST), "standardized diagnostic tools like PCR-based assays are essential for ensuring consistency and accuracy in infectious disease detection" (NIST, 2023). The RSV detection kit aligns with these principles, offering a reliable solution for both routine and emergency scenarios.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, with a strong focus on molecular biology and infectious disease testing. The company has established itself as a trusted name in the industry, providing innovative solutions that meet global standards.
Founded with a commitment to scientific excellence and patient care, HEBEI YOWIN has invested heavily in research and development to create products that address critical healthcare challenges. Their expertise in PCR technology and diagnostic kit design has enabled them to develop tools like the RSV detection kit, which are widely used in clinical and research settings.
The company's dedication to quality is reflected in its adherence to international standards such as ISO 13485, which ensures that its products meet the highest levels of safety and performance. This commitment to quality is further reinforced by rigorous validation processes for all its diagnostic solutions.
Advantages and Competitive Edge
The Respiratory Syncytial Virus Detection Kit offers several advantages over traditional diagnostic methods, making it a preferred choice for healthcare professionals:
- High Accuracy: The use of real-time PCR technology minimizes the risk of false negatives and positives, ensuring reliable results.
- Speed and Efficiency: The rapid workflow allows for quick diagnosis, which is critical in managing RSV infections and preventing their spread.
- Cost-Effectiveness: By reducing the need for multiple tests and minimizing the time required for diagnosis, the kit helps lower overall healthcare costs.
- Scalability: The kit's design allows for high-throughput testing, making it suitable for large-scale screening programs.
These advantages position the RSV detection kit as a valuable asset in both clinical and public health contexts. Its ability to deliver consistent performance and user-friendly operation makes it an ideal choice for laboratories of all sizes.
Integration with NIST Standards
The development of the RSV detection kit aligns with the NIST guidelines for diagnostic testing, which emphasize the importance of standardization, accuracy, and traceability. NIST's role in establishing reference materials and protocols for molecular diagnostics ensures that products like the RSV detection kit meet the highest standards of quality and reliability.
As noted by NIST, "the integration of standardized methodologies into diagnostic tools is essential for improving the consistency and reproducibility of test results across different laboratories" (NIST, 2023). The RSV detection kit exemplifies this principle, offering a solution that is both scientifically robust and practically applicable.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) represents a significant advancement in the field of molecular diagnostics. Its combination of high sensitivity, rapid results, and user-friendly design makes it an indispensable tool for healthcare professionals. By leveraging cutting-edge PCR technology and adhering to global standards, the kit ensures accurate and reliable RSV detection in a variety of clinical settings.
For more information about the product, visit the Cowingene Respiratory Syncytial Virus Detection Kit page. To learn more about the company behind this innovation, explore the HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. website.
Product Images
References
National Institute of Standards and Technology (NIST). (2023). Standardization in Diagnostic Testing. Retrieved from https://www.nist.gov/
