respiratory panel test for RSV-HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|Respiratory Pathogen Panel PCR
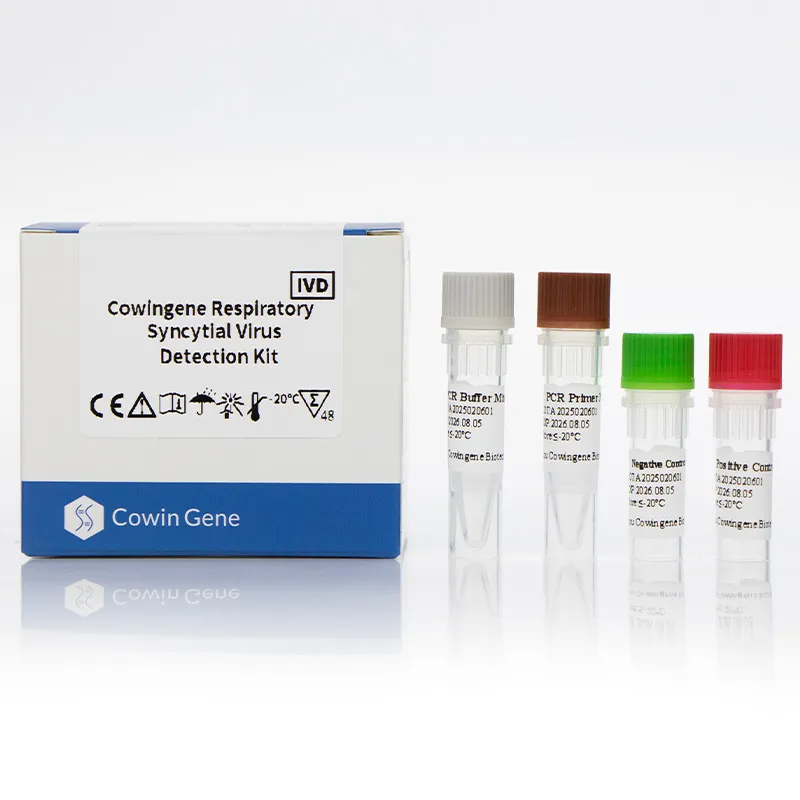
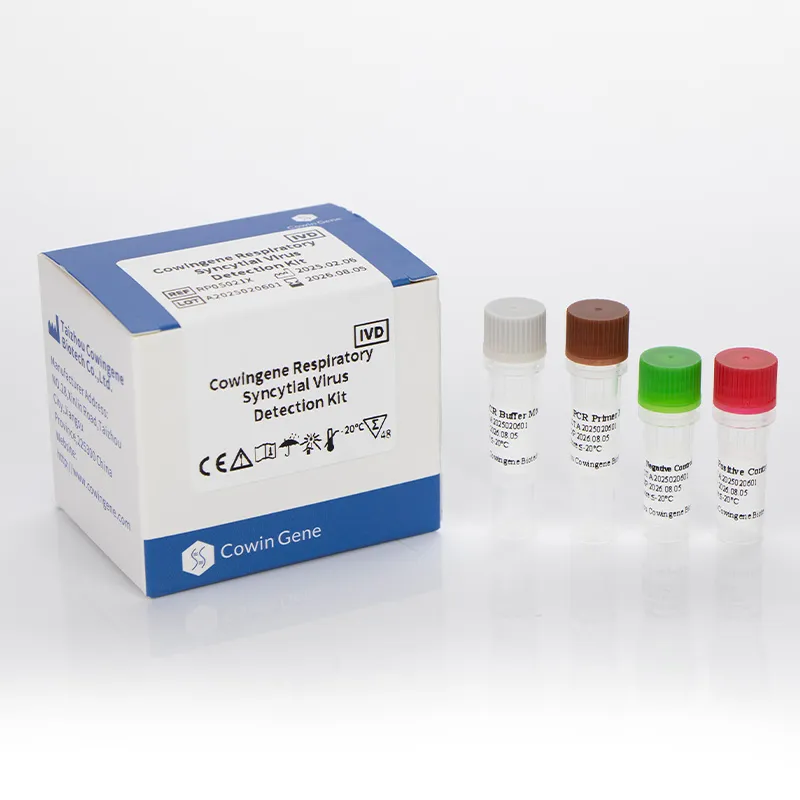
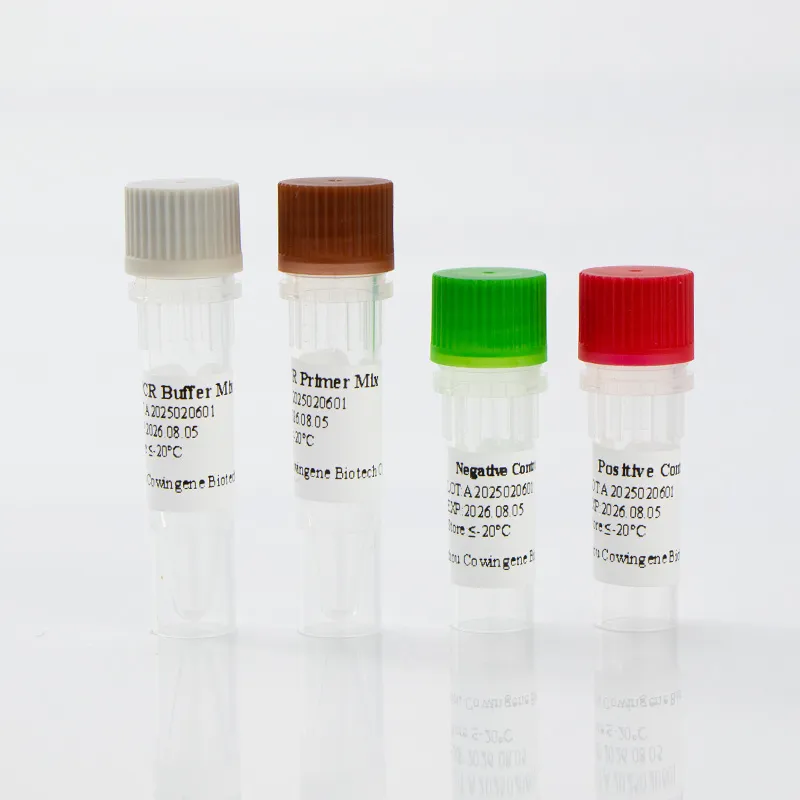
Cowingene Respiratory Syncytial Virus (RSV) Detection Kit (Liquid) represents a cutting-edge solution for rapid and accurate identification of respiratory syncytial virus (RSV) in clinical settings. Designed for use with validated specimen types such as nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, this kit leverages advanced PCR technology to deliver reliable results. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., a leader in medical diagnostics, the product combines precision engineering with user-friendly design to meet the demands of modern healthcare systems.
Product Overview
The Cowingene RSV Detection Kit (Liquid) is a molecular diagnostic tool specifically engineered for the detection of respiratory syncytial virus (RSV), a common cause of respiratory infections in infants, young children, and immunocompromised individuals. The kit is part of a broader respiratory pathogen panel solution, offering clinicians a comprehensive approach to diagnosing viral respiratory pathogens. Its respiratory panel test for RSV ensures high sensitivity and specificity, making it an essential tool in clinical laboratories and point-of-care settings.
Key Features and Advantages
1. High Sensitivity and Specificity
The kit employs polymerase chain reaction (PCR) technology to amplify and detect RSV genetic material with exceptional accuracy. This ensures minimal false-negative or false-positive results, which is critical for timely and effective patient management.
2. Rapid and Efficient Workflow
Designed for respiratory panel test for RSV, the kit streamlines the diagnostic process. Its liquid format simplifies handling and reduces the risk of cross-contamination, enabling laboratories to process samples efficiently even during high-volume periods.
3. Broad Sample Compatibility
Validated for use with nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, the kit accommodates a wide range of clinical specimen types. This versatility ensures adaptability across diverse healthcare environments.
4. User-Friendly Design
With clear instructions and intuitive protocols, the kit is optimized for both experienced and novice laboratory personnel. Its respiratory pathogen panel pcr workflow minimizes the need for specialized training, enhancing operational efficiency.
Technical Specifications
| Parameter | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Sample Type | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Technology | Real-Time PCR |
| Assay Format | Liquid-based |
| Detection Method | Fluorescent Reporter Probes |
| Run Time | Approximately 2 hours (including sample preparation) |
| Storage Conditions | -20°C for 12 months (unopened); 4°C for 1 month (opened) |
| Shipping | Blue ice or dry ice |
Applications and Use Cases
The Cowingene RSV Detection Kit (Liquid) is ideal for a variety of applications, including:
- Diagnostic Testing: Rapid identification of RSV in patients presenting with symptoms of respiratory infections, such as coughing, wheezing, and fever.
- Epidemiological Surveillance: Monitoring RSV outbreaks in community and hospital settings to inform public health strategies.
- Research and Development: Supporting studies on RSV pathogenesis, vaccine development, and antiviral therapies.
- Point-of-Care Testing: Enabling decentralized testing in clinics, urgent care centers, and remote locations.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of respiratory panel test for diagnostic solutions, dedicated to advancing healthcare through innovation. With a focus on respiratory pathogen panel pcr technologies, the company has established itself as a trusted partner for laboratories and healthcare providers worldwide. Their commitment to quality and precision is reflected in the Cowingene RSV Detection Kit (Liquid), which adheres to stringent international standards.
As a company rooted in respiratory panel test for RSV, HEBEI YOWIN leverages its expertise to address the evolving challenges of respiratory disease detection. Their products are designed to meet the needs of both developed and emerging markets, ensuring accessibility and reliability in diagnostic testing.
Compliance and Standards
The Cowingene RSV Detection Kit (Liquid) is developed in accordance with respiratory pathogen panel pcr industry standards, ensuring compliance with global regulatory requirements. The kit undergoes rigorous validation to guarantee performance consistency and safety. For more information on the product's compliance with NIST (National Institute of Standards and Technology) guidelines, refer to the NIST website.
NIST plays a critical role in establishing standards for medical diagnostics, including molecular testing technologies. While specific NIST guidelines for RSV detection may not be publicly available, the principles of accuracy, reliability, and traceability upheld by NIST are integral to the development of diagnostic tools like the Cowingene RSV kit.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) exemplifies the synergy of advanced technology and clinical expertise. With its respiratory panel test for RSV, the kit offers a reliable, efficient, and user-friendly solution for respiratory pathogen detection. As healthcare systems worldwide face increasing demands for rapid and accurate diagnostics, products like this play a vital role in improving patient outcomes and public health responses.
For more details about the product, visit the Cowingene RSV Detection Kit (Liquid) page or explore the HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. website to learn about their comprehensive range of diagnostic solutions.
References
NIST (National Institute of Standards and Technology). (n.d.). https://www.nist.gov/
Note: While the specific NIST page referenced in the context could not be retrieved, the principles of measurement and standardization upheld by NIST are foundational to the development of diagnostic technologies like the Cowingene RSV Detection Kit.
