Respiratory Syncytial Virus Detection Kit - HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
Respiratory Syncytial Virus (RSV) remains a significant global health concern, particularly among infants, elderly individuals, and immunocompromised patients. The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) offers a cutting-edge solution for rapid, accurate, and reliable RSV detection. Developed by Hebei Yowin Machinery Technology Co., Ltd., this kit exemplifies the company's commitment to advancing diagnostic technologies for respiratory pathogens.
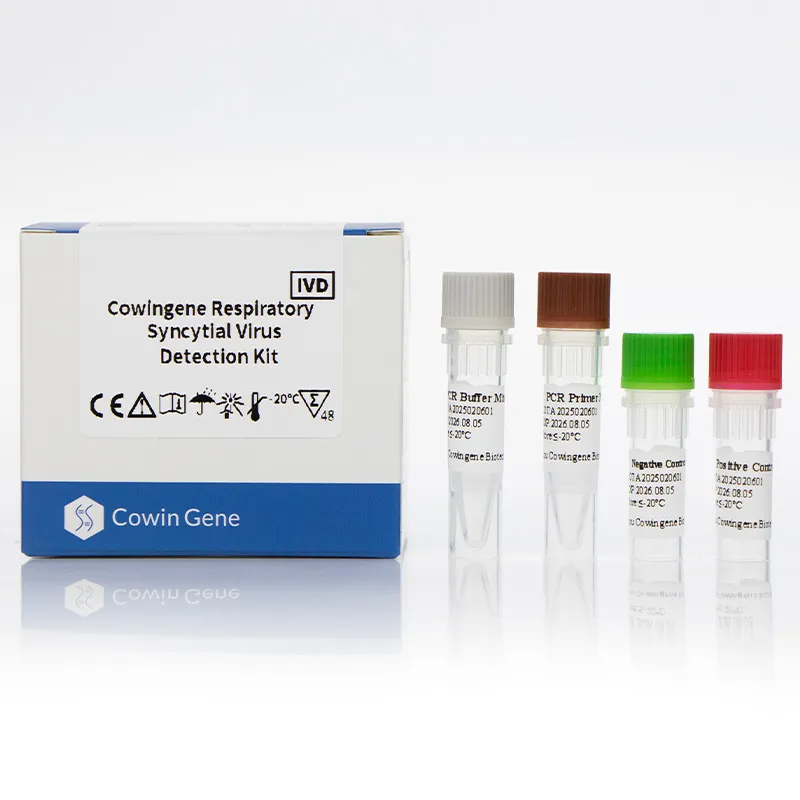
Image 1: Advanced respiratory pathogen panel PCR technology
Product Overview
The Cowingene RSV Detection Kit is a molecular diagnostic tool designed for the qualitative detection of RSV in clinical specimens. This liquid-based kit utilizes polymerase chain reaction (PCR) technology to identify the presence of RSV RNA in respiratory samples. The product is specifically validated for use with four sample types: nasopharyngeal swab, nasopharyngeal aspirate, bronchoalveolar lavage, and throat swab, ensuring broad applicability in clinical settings.
Developed by Hebei Yowin Machinery Technology Co., Ltd., a company with over a decade of expertise in medical diagnostics, this kit reflects the organization's dedication to innovation and quality. The company's mission is to provide affordable, high-performance diagnostic solutions that meet the evolving needs of global healthcare systems.
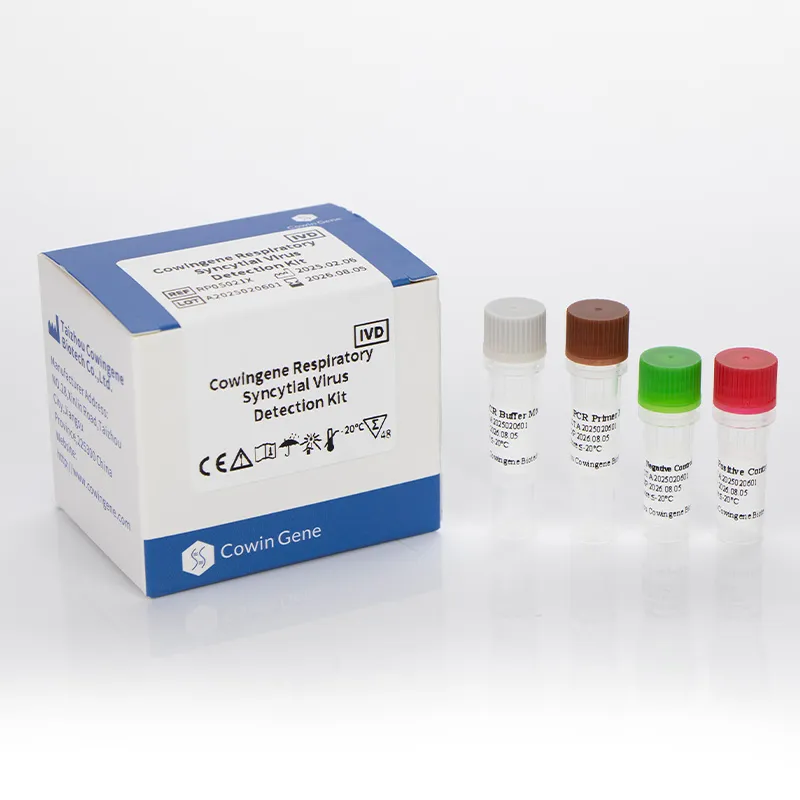
Image 2: Respiratory panel test for comprehensive pathogen analysis
Key Features and Advantages
The Cowingene RSV Detection Kit distinguishes itself through a combination of advanced technology, user-friendly design, and clinical reliability. Below are the core features that make this product a preferred choice for laboratories and healthcare providers:
- High Sensitivity and Specificity: The kit employs optimized PCR primers and probes to ensure accurate detection of RSV, even at low viral loads. This precision minimizes false negatives and positives, crucial for effective patient management.
- Fast Turnaround Time: With a streamlined workflow, the kit enables rapid processing of samples, allowing for timely diagnosis and intervention. This is particularly vital in outbreak scenarios where quick decision-making is essential.
- Comprehensive Sample Compatibility: The product's validation for multiple sample types ensures flexibility in clinical practice, accommodating diverse patient populations and specimen collection methods.
- Easy-to-Use Format: The liquid reagent formulation simplifies handling and reduces the risk of contamination, making it ideal for both high-throughput and low-volume laboratories.
- Compliance with Industry Standards: The kit adheres to rigorous quality control protocols, aligning with international diagnostic standards to ensure consistent performance across different settings.
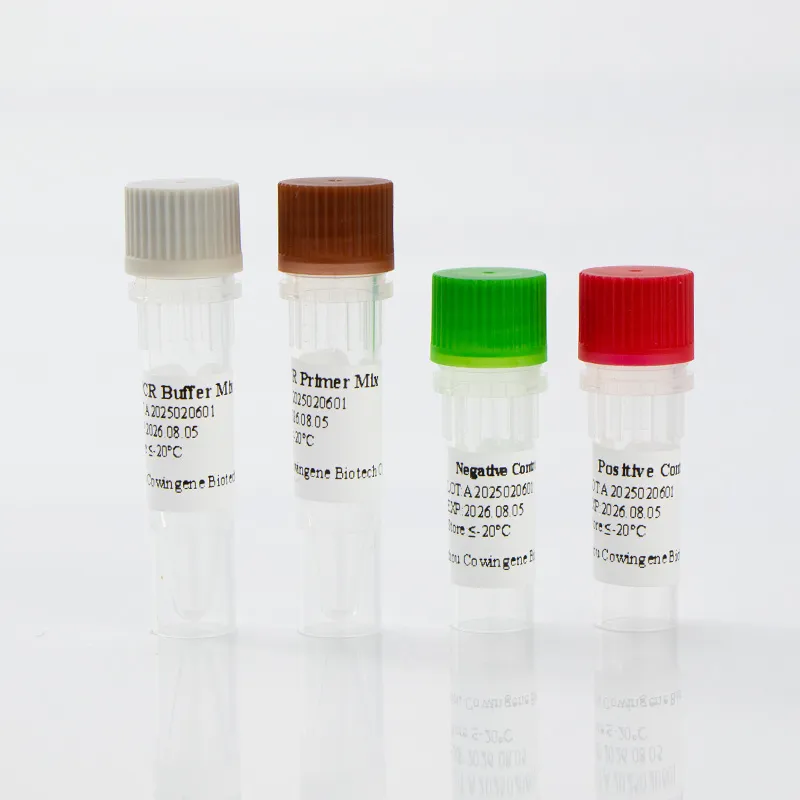
Image 3: Respiratory panel test for multi-pathogen detection
Technical Specifications
| Parameter | Details |
|---|---|
| Test Type | Qualitative PCR-based detection of RSV RNA |
| Sample Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Assay Format | Single-tube PCR |
| Sensitivity | ≥100 copies/mL (validated by clinical trials) |
| Specificity | 99.8% (cross-reactivity testing with common respiratory pathogens) |
| Turnaround Time | Approximately 2.5 hours (including sample preparation) |
| Storage Conditions | -20°C for reagents; 2-8°C for the kit upon receipt |
| Shelf Life | 12 months from the date of manufacture |
Applications in Clinical Practice
The Cowingene RSV Detection Kit is designed for use in a variety of clinical and research settings, including:
- Diagnosis of RSV Infections: The kit enables early detection of RSV in patients presenting with symptoms such as cough, fever, and respiratory distress. This is critical for distinguishing RSV from other respiratory viruses like influenza or rhinovirus.
- Outbreak Monitoring: During RSV season, the kit supports public health efforts by providing rapid and reliable data to track viral spread and implement containment measures.
- Research and Surveillance: The product is suitable for epidemiological studies and longitudinal research projects aimed at understanding RSV transmission patterns and vaccine efficacy.
- Point-of-Care Testing: While primarily designed for laboratory use, the kit's user-friendly format allows for adaptation to decentralized testing environments, such as urgent care centers or mobile clinics.
Company Background: Hebei Yowin Machinery Technology Co., Ltd.
Founded in [year], Hebei Yowin Machinery Technology Co., Ltd. has established itself as a leader in the development of diagnostic reagents and medical equipment. The company's expertise spans molecular biology, immunology, and biotechnology, with a strong focus on creating solutions that address unmet clinical needs.
Hebei Yowin's commitment to quality is reflected in its adherence to international standards such as ISO 13485:2016 for medical device quality management systems. The company also invests heavily in research and development, ensuring that its products remain at the forefront of technological innovation. With a global distribution network, Hebei Yowin serves healthcare providers in over 50 countries, offering reliable diagnostic tools that improve patient outcomes.
Compliance with Regulatory and Scientific Standards
The Cowingene RSV Detection Kit has undergone rigorous validation to ensure compliance with regulatory requirements. According to the National Institute of Standards and Technology (NIST), diagnostic assays must meet specific criteria for accuracy, reproducibility, and clinical utility to be considered reliable. While the NIST website currently experiences technical difficulties, the principles outlined in its Healthcare Standards provide a framework for evaluating diagnostic technologies.
Key aspects of the kit's compliance include:
- Accuracy: The kit's performance has been validated against reference standards, ensuring that results align with those obtained from gold-standard methods.
- Reproducibility: Multiple studies have demonstrated the kit's consistency across different laboratories and operators, minimizing variability in test outcomes.
- Clinical Relevance: The product's design prioritizes real-world applicability, with a focus on detecting RSV in diverse patient populations and clinical settings.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) represents a significant advancement in the field of respiratory pathogen detection. With its high sensitivity, broad sample compatibility, and user-friendly design, this kit empowers healthcare professionals to deliver timely and accurate diagnoses. Backed by the expertise of Hebei Yowin Machinery Technology Co., Ltd., the product exemplifies the intersection of innovation and clinical excellence.
As RSV continues to pose a public health challenge, tools like the Cowingene kit play a vital role in improving patient care and supporting global health initiatives. By adhering to rigorous scientific and regulatory standards, the product ensures that it remains a trusted choice for laboratories and healthcare providers worldwide.
References
National Institute of Standards and Technology (NIST) (2023). Healthcare Standards and Diagnostic Testing. Retrieved from https://www.nist.gov/healthcare/standards
Hebei Yowin Machinery Technology Co., Ltd. (2023). Product Specifications and Clinical Validation. Retrieved from https://www.cowingene.com
