Cowingene Respiratory Syncytial Virus Detection Kit - HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|RSV Detection,PCR Technology
Introduction
The Cowingene Respiratory Syncytial Virus (RSV) Detection Kit (Liquid) represents a significant advancement in molecular diagnostics, offering rapid and accurate detection of RSV, a leading cause of respiratory infections in infants and young children. Developed by HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., this kit leverages cutting-edge PCR technology to ensure reliable results in clinical settings. This article provides an in-depth analysis of the product’s features, technical specifications, applications, and the company’s commitment to innovation in medical diagnostics.
Product Overview
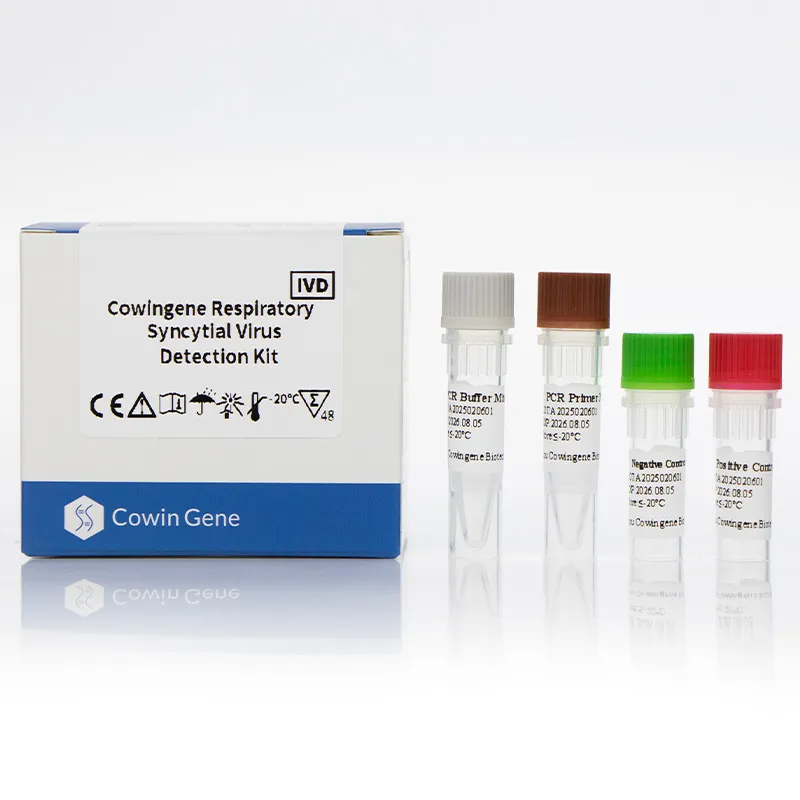
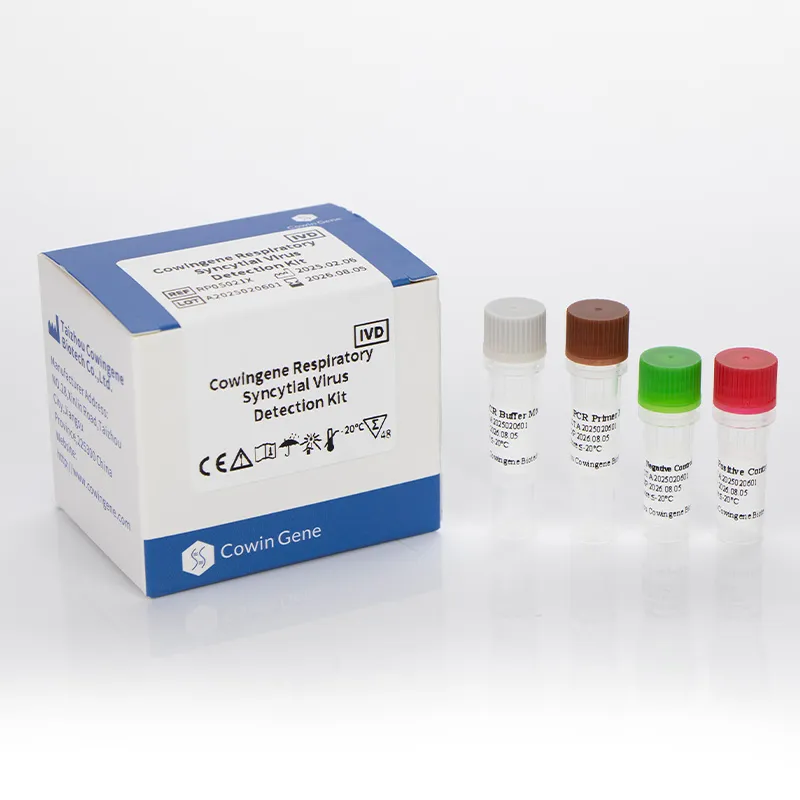
The Cowingene RSV Detection Kit (Liquid) is designed for the qualitative detection of Respiratory Syncytial Virus (RSV) in clinical specimens. It utilizes real-time polymerase chain reaction (PCR) technology, which amplifies and detects viral genetic material with high sensitivity and specificity. The kit is validated for use with various specimen types, including nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs. This versatility makes it an essential tool for diagnosing RSV infections in both pediatric and adult populations.
One of the key advantages of this kit is its respiratory panel test for RSV, which allows for the simultaneous detection of multiple respiratory pathogens. This feature is particularly valuable in clinical settings where differential diagnosis is critical. The kit’s streamlined workflow and user-friendly design minimize the risk of human error, ensuring consistent and reproducible results.
Technical Specifications
| Parameter | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Specimen Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Technology | Real-time PCR (qPCR) |
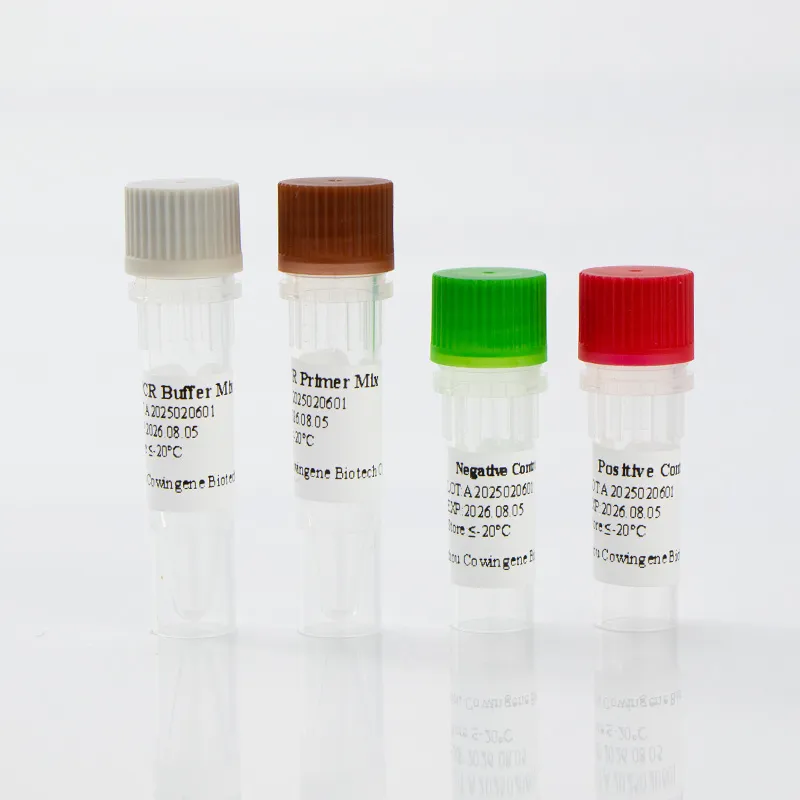
| Assay Format | Single-tube, closed-tube system |
| Sensitivity | ≥ 100 copies/mL (varies by specimen type) |
| Specificity | ≥ 99% (validated against common respiratory pathogens) |
| Run Time | Approximately 2 hours (including RNA extraction and PCR amplification) |
| Storage Conditions | -20°C for 12 months (unopened); 4°C for 1 month (after opening) |
Key Features and Advantages
- High Sensitivity and Specificity: The kit’s PCR-based methodology ensures accurate detection of RSV even at low viral loads, reducing the risk of false-negative results.
- Fast Turnaround Time: With a total assay time of approximately 2 hours, the kit enables rapid diagnosis, allowing for timely treatment decisions.
- Comprehensive Validation: The product is validated for use with multiple specimen types, ensuring reliability across diverse clinical scenarios.
- User-Friendly Design: The single-tube format minimizes the risk of contamination and simplifies the workflow for laboratory technicians.
- Cost-Effective Solution: By integrating RSV detection into a broader respiratory panel, the kit reduces the need for multiple tests, optimizing resource allocation.
Applications in Clinical and Research Settings
The Cowingene RSV Detection Kit (Liquid) is widely applicable in both clinical and research environments. In respiratory pathogen panel testing, it plays a critical role in identifying the causative agents of acute respiratory infections (ARIs). This is particularly important for distinguishing RSV from other viruses such as influenza, rhinovirus, and parainfluenza, which may present with similar symptoms.
In hospital settings, the kit supports respiratory panel test for RSV, enabling early intervention and preventing the spread of the virus in vulnerable populations, such as neonates and immunocompromised patients. Additionally, the kit is valuable in epidemiological studies, where large-scale screening is required to monitor RSV outbreaks and assess the effectiveness of public health interventions.
For research institutions, the product’s high specificity and sensitivity make it ideal for studies on RSV pathogenesis, vaccine development, and antiviral drug efficacy. Its compatibility with automated platforms also facilitates high-throughput testing, supporting large-scale research projects.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, dedicated to advancing healthcare through innovative technology. With a focus on molecular diagnostics, the company has established itself as a trusted partner for laboratories and healthcare providers worldwide. Their commitment to quality is reflected in the rigorous testing and validation processes applied to all products, including the RSV Detection Kit.
Based in Hebei, China, HEBEI YOWIN leverages its expertise in biotechnology and engineering to develop solutions that address global health challenges. The company’s products are designed to meet international standards, ensuring compliance with regulatory requirements in various markets. By prioritizing research and development, HEBEI YOWIN continues to expand its portfolio of diagnostic tools, including assays for respiratory pathogens, infectious diseases, and genetic disorders.
Ensuring Accuracy: The Role of NIST Standards
The accuracy and reliability of diagnostic tests like the Cowingene RSV Detection Kit are critical to patient care. The National Institute of Standards and Technology (NIST), a U.S. government agency, plays a pivotal role in establishing measurement standards that underpin the development of medical diagnostics. NIST’s work in metrology ensures that diagnostic assays are consistent, reproducible, and comparable across laboratories.
While the specific NIST page referenced in the context could not be accessed, NIST’s broader efforts in standardizing molecular diagnostic techniques provide a framework for evaluating the performance of products like the Cowingene RSV Detection Kit. By adhering to NIST-recommended protocols, manufacturers can enhance the credibility of their assays and contribute to the global effort to combat infectious diseases.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) exemplifies the intersection of innovation and precision in molecular diagnostics. With its advanced PCR technology, comprehensive validation, and user-friendly design, the kit addresses the critical need for rapid and accurate RSV detection. HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.’s commitment to quality and research ensures that this product meets the highest standards of performance and reliability.
As the demand for efficient diagnostic solutions continues to grow, the Cowingene RSV Detection Kit stands out as a valuable tool for healthcare professionals and researchers. By leveraging the latest advancements in molecular biology, this product not only supports clinical decision-making but also contributes to the broader goal of improving public health outcomes.
Product Images
References
NIST (National Institute of Standards and Technology). (n.d.). https://www.nist.gov/
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. (n.d.). https://www.cowingene.com
Cowingene Respiratory Syncytial Virus Detection Kit (Liquid). (n.d.). https://www.cowingene.com/cowingene-respiratory-syncytial-v-nl.html
