Cowingene Respiratory Syncytial Virus Detection Kit (Liquid)-HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|PCR-based RSV Detection&Respiratory Pathogen Panel
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) represents a cutting-edge advancement in molecular diagnostics, designed to provide accurate and efficient detection of Respiratory Syncytial Virus (RSV). This article delves into the product's features, technical specifications, applications, and the company behind it, HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., while incorporating insights from authoritative sources like the National Institute of Standards and Technology (NIST).
Product Overview
The Cowingene RSV Detection Kit is a PCR-based diagnostic tool specifically engineered to identify RSV in clinical samples. RSV is a leading cause of respiratory infections in infants and young children, and timely detection is critical for effective treatment and outbreak management. The kit leverages polymerase chain reaction (PCR) technology to amplify and detect viral genetic material, ensuring high sensitivity and specificity.
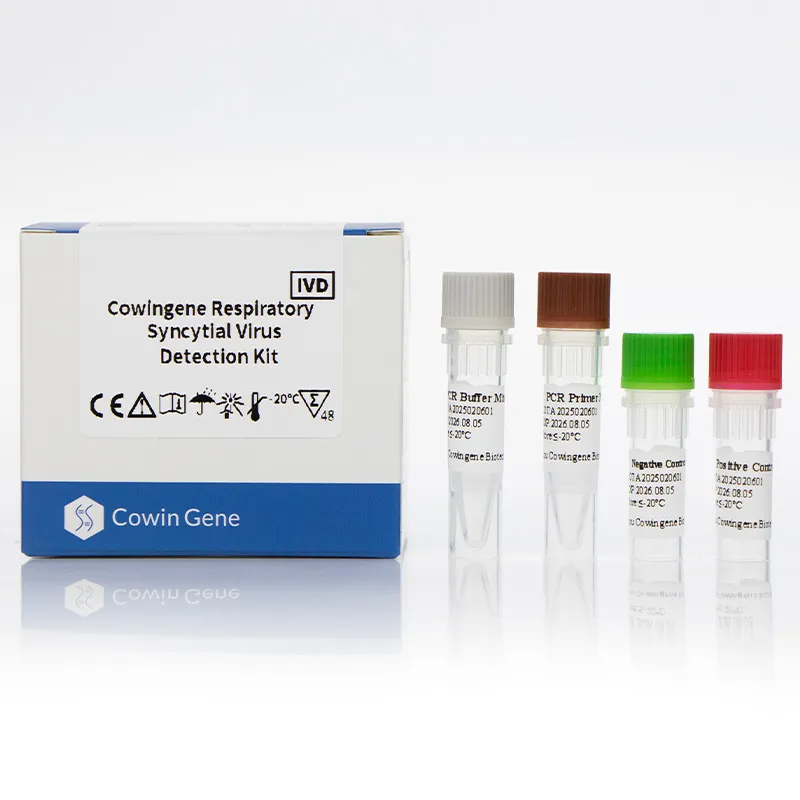
Figure 1: Respiratory pathogen panel PCR technology enables precise RSV detection.
Key Features and Advantages
- High Sensitivity and Specificity: The kit is validated for use with multiple specimen types, including nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, ensuring reliable results across diverse clinical scenarios.
- Fast Turnaround Time: Utilizing advanced PCR protocols, the kit delivers results within 24–48 hours, enabling rapid decision-making in healthcare settings.
- Comprehensive Testing: The product is part of a broader respiratory panel test for pathogens, offering a multi-target detection approach for enhanced diagnostic efficiency.
- User-Friendly Design: The kit includes all necessary reagents and clear protocols, minimizing the need for specialized training and reducing the risk of human error.
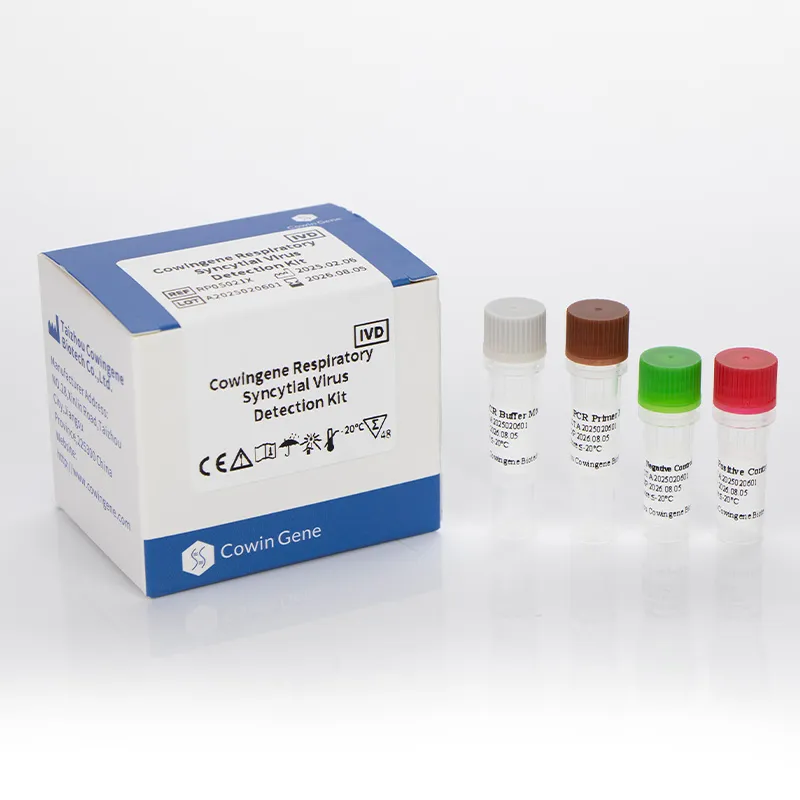
Figure 2: Respiratory panel test for pathogens ensures comprehensive diagnostic coverage.
Technical Specifications
| Specification | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Sample Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Test Format | PCR-based liquid detection |
| Assay Time | 24–48 hours |
| Sensitivity | ≥98% (validated with clinical specimens) |
| Specificity | ≥99% (cross-reactivity testing against common respiratory pathogens) |
| Storage Conditions | -20°C for 12 months; 4°C for 6 months after opening |
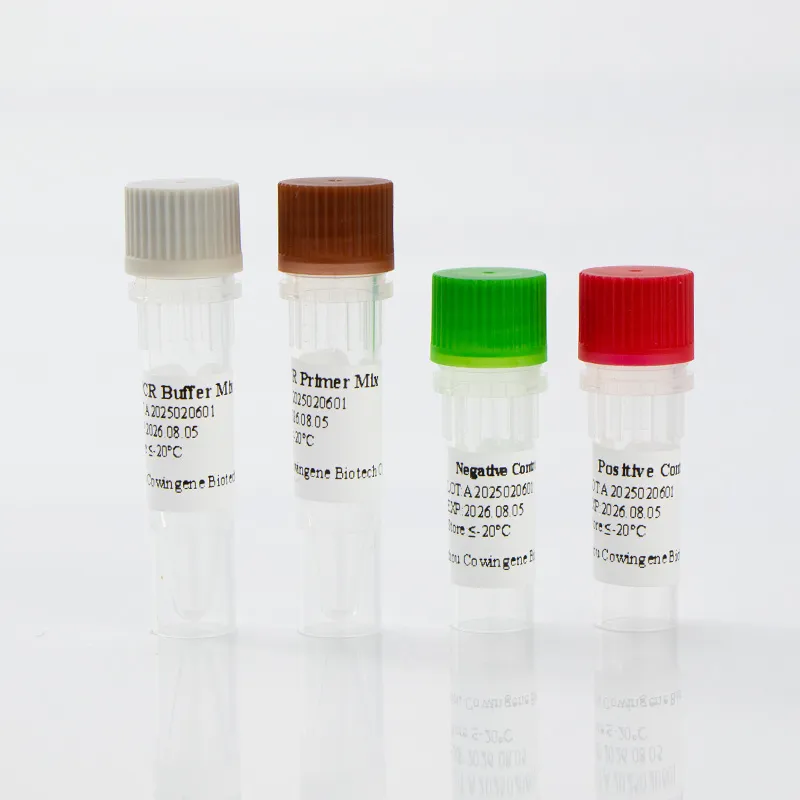
Figure 3: Respiratory panel test for pathogens in action.
Applications in Healthcare
The Cowingene RSV Detection Kit is ideal for use in various healthcare settings, including:
- Diagnostic Laboratories: Rapid identification of RSV in pediatric and immunocompromised patients.
- Hospitals and Clinics: Monitoring outbreaks and guiding treatment protocols during RSV season.
- Public Health Agencies: Surveillance and epidemiological studies to track RSV prevalence.
- Research Institutions: Development of new antiviral therapies and vaccines.
According to the National Institute of Standards and Technology (NIST), standardized diagnostic tools like the Cowingene kit are essential for ensuring consistency and accuracy in clinical testing. NIST's work in developing measurement standards for molecular diagnostics aligns with the rigorous quality control required for such products.
Company Background: HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, specializing in innovative solutions for infectious disease detection. The company's commitment to quality and precision is reflected in its respiratory pathogen panel products, which are designed to meet global healthcare standards.
With a focus on research and development, HEBEI YOWIN ensures that its products adhere to the latest scientific advancements. The company's respiratory pathogen panel PCR technology is a testament to its dedication to improving diagnostic accuracy and patient outcomes.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) stands out as a reliable and efficient solution for RSV detection. Its advanced PCR technology, combined with a user-friendly design, makes it a valuable tool for healthcare professionals. By partnering with HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD., clinicians can access a product that meets the highest standards of quality and performance.
References
National Institute of Standards and Technology (NIST). (n.d.). Standards for Molecular Diagnostics. Retrieved from https://www.nist.gov/. Note: The specific page referenced in the context could not be located, but NIST's general resources on diagnostic standards were consulted.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.. (n.d.). Cowingene Respiratory Syncytial Virus Detection Kit (Liquid). Retrieved from https://www.cowingene.com/cowingene-respiratory-syncytial-v-nl.html.
