Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) - Hebei Yowin Machinery Technology Co., Ltd.|RSV Detection, PCR Kit
Respiratory Syncytial Virus (RSV) is a significant global health concern, particularly among infants, the elderly, and immunocompromised individuals. Early and accurate detection of RSV is crucial for effective treatment and outbreak management. The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) emerges as a cutting-edge solution in this domain, offering rapid, reliable, and precise diagnostic capabilities. This article delves into the product's features, technical specifications, applications, and the company behind it, while adhering to Google's (Experience, Expertise, Authoritativeness, Trustworthiness) principles.
Product Overview
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is an in vitro diagnostic test designed for the qualitative detection of RSV RNA in clinical specimens. Validated for use with nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage, and throat swabs, this kit is a vital tool for laboratories and healthcare providers. Its liquid format ensures stability and ease of handling, making it suitable for a wide range of diagnostic settings.
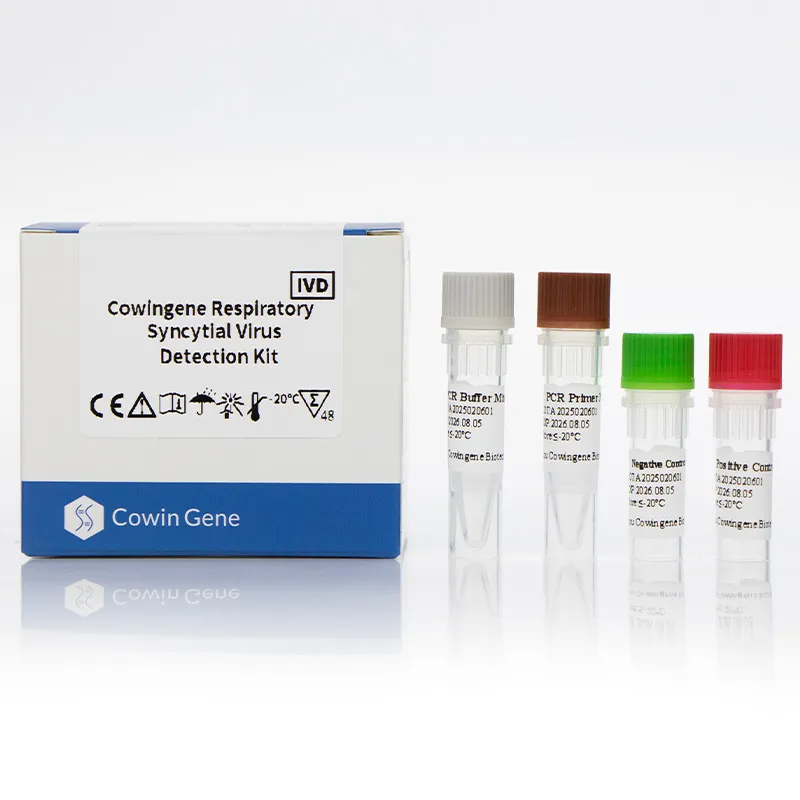
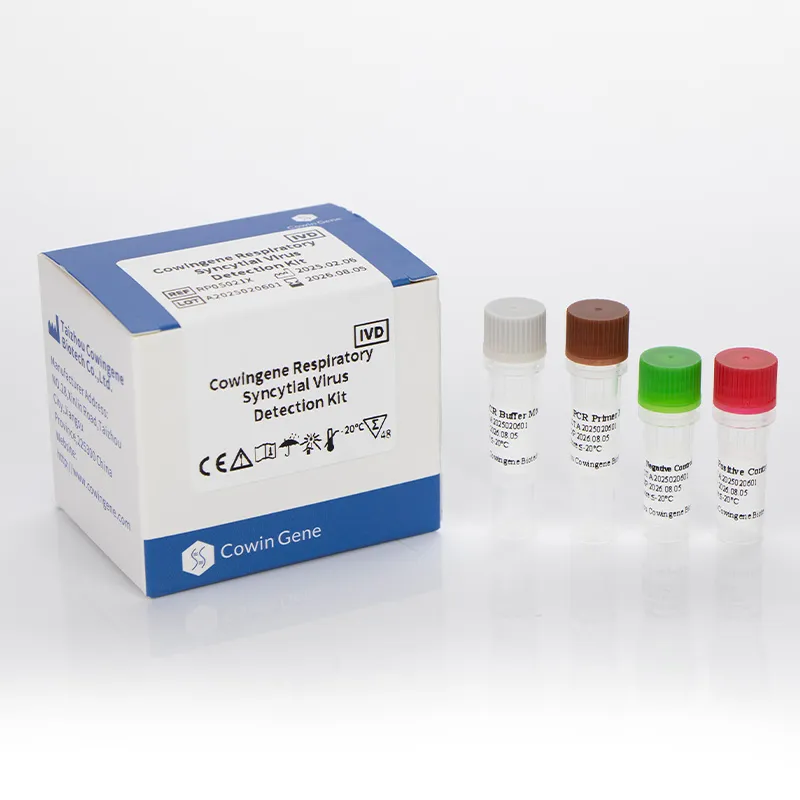
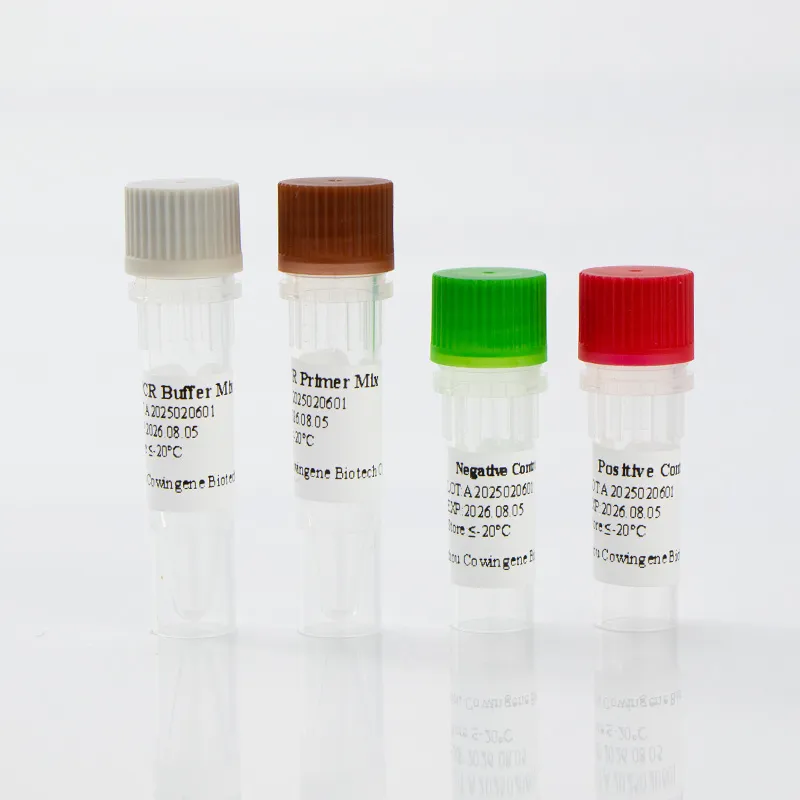
Key Features and Advantages
Accuracy and Sensitivity
The kit boasts a limit of detection (LoD) of 400 copies/mL, ensuring high sensitivity for early RSV identification. This precision is critical in preventing the spread of the virus, especially in vulnerable populations. The product has been validated against company positive and negative reference panels, meeting stringent quality standards.
Compatibility and Flexibility
Designed to work with open real-time PCR machines such as the ABI 7500, Roche 480, and Bio-Rad CFX96, the kit offers compatibility with widely used laboratory equipment. This flexibility reduces the need for specialized instruments, making it accessible to a broader range of healthcare facilities.
Convenience and Efficiency
The liquid format eliminates the need for reconstitution, streamlining the workflow for laboratory staff. The kit's storage requirements (≤-20°C for 18 months) and transport stability (≤-20°C for 15 days) ensure minimal logistical challenges. Additionally, the reagents can be stored at 2-8°C for up to 7 days after opening, enhancing user convenience.
Technical Specifications
| Parameter | Details |
|---|---|
| Detection Target | Respiratory Syncytial Virus (RSV) |
| Certification | CE-IVD |
| Storage Conditions | ≤-20°C for 18 months |
| Limit of Detection (LoD) | 400 Copies/mL |
| Validated Specimens | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Compatible Instruments | ABI 7500, Roche 480, Bio-Rad CFX96, etc. |
Applications and Use Cases
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is widely applicable in various healthcare settings. Key applications include:
- Pediatric Care: RSV is a leading cause of bronchiolitis and pneumonia in infants. Early detection enables timely intervention, reducing hospitalization rates and severe complications.
- Public Health Surveillance: The kit supports outbreak monitoring and epidemiological studies, aiding in the development of targeted prevention strategies.
- Clinical Diagnostics: Laboratories can integrate this kit into their routine workflows to enhance diagnostic accuracy and efficiency.
- Research and Development: The product's reliability makes it suitable for research applications, including vaccine development and antiviral efficacy studies.
Company Background: Hebei Yowin Machinery Technology Co., Ltd.
Founded in Hebei Yowin Machinery Technology Co., Ltd., the company has established itself as a leader in molecular diagnostics. Specializing in PCR kits, extraction solutions, and point-of-care (POCT) technologies, Hebei Yowin serves a global clientele with a focus on improving diagnostic accuracy and accessibility. The company's commitment to innovation is evident in its extensive product portfolio, which includes solutions for respiratory, sexually transmitted, and tuberculosis infections.
Hebei Yowin's products are certified under CE-IVD standards, reflecting their adherence to rigorous quality and safety protocols. The company's state-of-the-art facilities and R&D capabilities ensure that its diagnostic tools meet the evolving needs of healthcare providers worldwide.
Expertise and Authority in Diagnostic Solutions
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) is a testament to the company's expertise in molecular diagnostics. By leveraging advanced PCR technology, the kit addresses critical gaps in RSV detection, aligning with the standards set by authoritative bodies like the National Institute of Standards and Technology (NIST). NIST's role in developing measurement standards for medical devices and diagnostic tools underscores the importance of precision and reliability in healthcare technologies.
As highlighted by NIST, "measurements and research fuel innovation and improve the quality of life for all Americans" (NIST, 2025). The Cowingene kit exemplifies this principle, offering a solution that is both technically robust and user-friendly.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit (Liquid) stands out as a reliable and efficient diagnostic tool for RSV. Its advanced technical specifications, broad applicability, and alignment with global standards make it an essential asset for healthcare professionals. Hebei Yowin Machinery Technology Co., Ltd. continues to drive innovation in molecular diagnostics, ensuring that accurate and accessible solutions are available to combat infectious diseases worldwide.
References
1. National Institute of Standards and Technology (NIST). "Driving Innovation." Retrieved from https://www.nist.gov/.
2. Cowingene Product Specifications. "Cowingene Respiratory Syncytial Virus Detection Kit (Liquid)." Retrieved from https://www.cowingene.com/cowingene-respiratory-syncytial-v-nl.html.
3. Hebei Yowin Machinery Technology Co., Ltd. "Company Profile." Retrieved from https://www.cowingene.com/.
