Cowingene RSV Detection Kit - HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD.|Respiratory Pathogen Panel, PCR Testing
Introduction
The Cowingene Respiratory Syncytial Virus Detection Kit represents a significant advancement in molecular diagnostics, offering a reliable solution for detecting Respiratory Syncytial Virus (RSV) in clinical settings. This article explores the product's core features, technical specifications, applications, and the background of its manufacturer, HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD..
Product Overview
The Cowingene Respiratory Syncytial Virus Detection Kit is a liquid-based PCR test designed to detect RSV in respiratory specimens. This kit is part of a broader portfolio of respiratory pathogen panel solutions, catering to the growing demand for rapid and accurate diagnostic tools in healthcare.
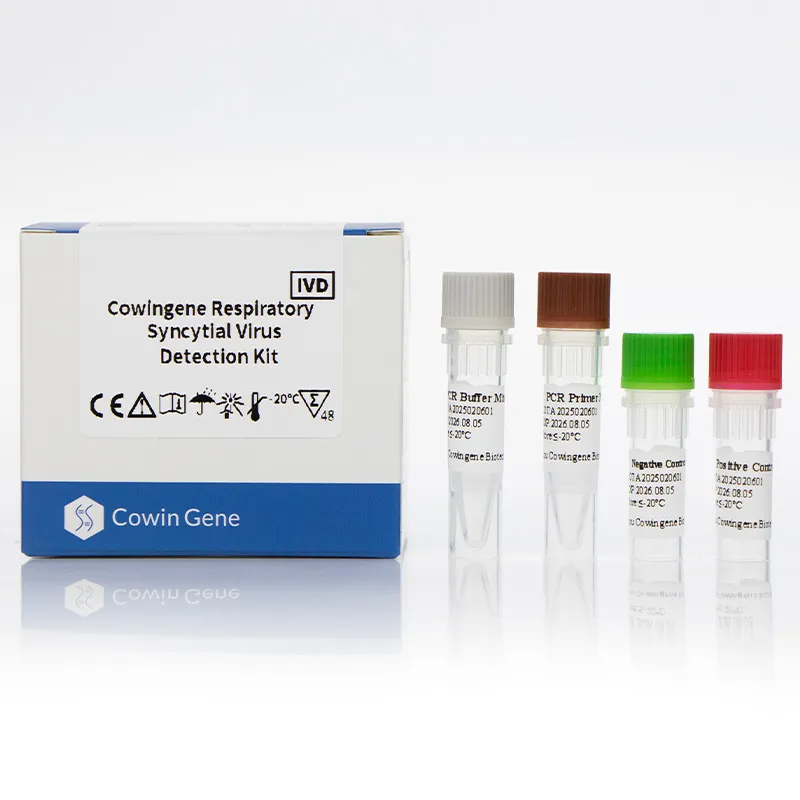
Key features include high specificity, minimal cross-reactivity, and compatibility with standard PCR platforms. The kit's design ensures ease of use, making it suitable for both clinical laboratories and point-of-care settings.
Technical Specifications
| Parameter | Details |
|---|---|
| Target Pathogen | Respiratory Syncytial Virus (RSV) |
| Sample Types | Nasopharyngeal swab, Nasopharyngeal aspirate, Bronchoalveolar lavage, Throat swab |
| Test Format | Real-Time PCR |
| Assay Type | Singleplex |
| Kit Components | 1 tube containing reagents for RSV detection |
| Storage Conditions | -20°C for up to 12 months |
Product Advantages
- High Sensitivity and Specificity: The kit employs advanced molecular techniques to ensure accurate detection of RSV, minimizing false positives and negatives.
- Fast Turnaround Time: Results are typically available within 2-3 hours, enabling timely clinical decisions.
- Comprehensive Testing: Part of the respiratory panel test for broader pathogen detection, allowing for multi-target analysis in a single run.
- User-Friendly Design: Simplified protocols reduce the risk of human error, making it accessible for both novice and experienced technicians.
Applications
The Cowingene RSV Detection Kit is widely used in various healthcare settings, including:
- Diagnostic Laboratories: For routine screening of respiratory infections, especially during RSV season.
- Public Health Surveillance: Monitoring outbreaks and tracking viral strains to inform public health strategies.
- Research Institutions: Supporting studies on RSV epidemiology, vaccine development, and antiviral therapies.
- Point-of-Care Testing: Enabling rapid diagnosis in clinics, emergency departments, and remote locations.
According to the National Institute of Standards and Technology (NIST), standardized diagnostic tools are critical for ensuring consistency and reliability in clinical testing. The Cowingene kit aligns with these principles, contributing to the broader goal of improving healthcare outcomes through precision diagnostics.
Company Background
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. is a leading manufacturer of diagnostic and medical equipment, specializing in innovative solutions for infectious disease detection. With a commitment to quality and technological advancement, the company has established itself as a trusted partner in the global healthcare industry.
HEBEI YOWIN's products are designed to meet international standards, ensuring compliance with regulatory requirements and user safety. Their focus on research and development has led to the creation of advanced diagnostic kits like the Cowingene Respiratory Syncytial Virus Detection Kit, which addresses the urgent need for rapid and accurate pathogen identification.
Industry Relevance and Future Prospects
Respiratory Syncytial Virus (RSV) is a major cause of lower respiratory tract infections, particularly in infants and the elderly. The Cowingene RSV Detection Kit plays a vital role in combating this public health challenge by providing clinicians with a reliable tool for early diagnosis.
As the demand for molecular diagnostics continues to grow, companies like HEBEI YOWIN are poised to lead the market with cutting-edge technologies. The integration of AI and automation in diagnostic workflows, as highlighted by NIST research, further underscores the importance of innovative solutions in improving healthcare efficiency and accuracy.
Conclusion
The Cowingene Respiratory Syncytial Virus Detection Kit exemplifies the synergy between advanced technology and clinical needs. With its robust design, ease of use, and alignment with industry standards, this product is a valuable asset for healthcare professionals worldwide. As the field of molecular diagnostics evolves, HEBEI YOWIN's commitment to innovation ensures that their solutions remain at the forefront of medical science.
References
National Institute of Standards and Technology (NIST) (2025). Standards for Diagnostic Technologies. Retrieved from https://www.nist.gov/.
HEBEI YOWIN MACHINERY TECHNOLOGY CO.,LTD. (2025). Cowingene Respiratory Syncytial Virus Detection Kit. Retrieved from https://www.cowingene.com/cowingene-respiratory-syncytial-v-nl.html.
